Volume 7, Issue 2 (December 2021)
Elderly Health Journal 2021, 7(2): 71-78 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
jafari M, Hosseinpour Delavar S, Safikhani H, Azizi M. The Effect of Eight Weeks of Continuous and Interval Training with Citrus Aurantium Consumption on Autophagy Markers and MyoD Activation in the Muscle Tissue of Elderly Rats. Elderly Health Journal 2021; 7 (2) :71-78
URL: http://ehj.ssu.ac.ir/article-1-226-en.html
URL: http://ehj.ssu.ac.ir/article-1-226-en.html
Department of Exercise Physiology, Kermanshah Branch, Islamic Azad University, Kermanshah, Iran , delavar2009@iauksh.ac.ir
Full-Text [PDF 1454 kb]
(411 Downloads)
| Abstract (HTML) (1041 Views)
Results
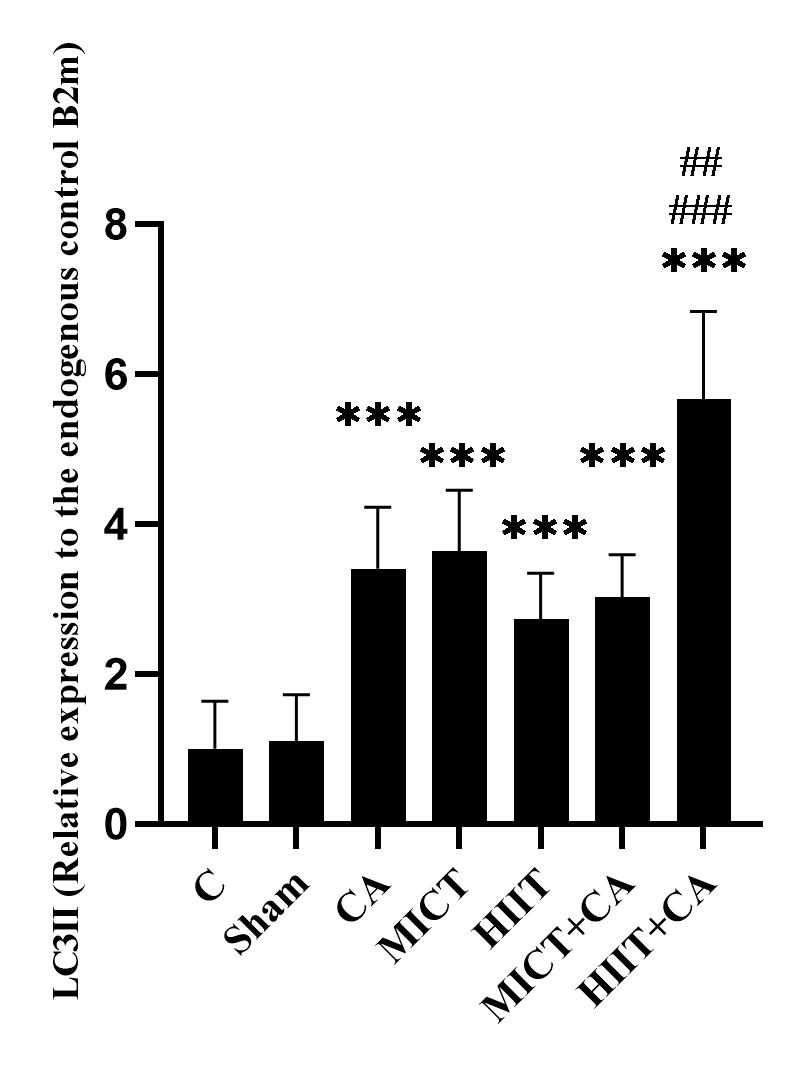
Figure 1. mRNA quantification of LC3II gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001) Significant increase compared to the C group.
### (p ≥ 0.001) Significant increase compared to the CA, MICT and MICT + CA groups
## (p ≥ 0.01) Significant increase compared to the MICT group
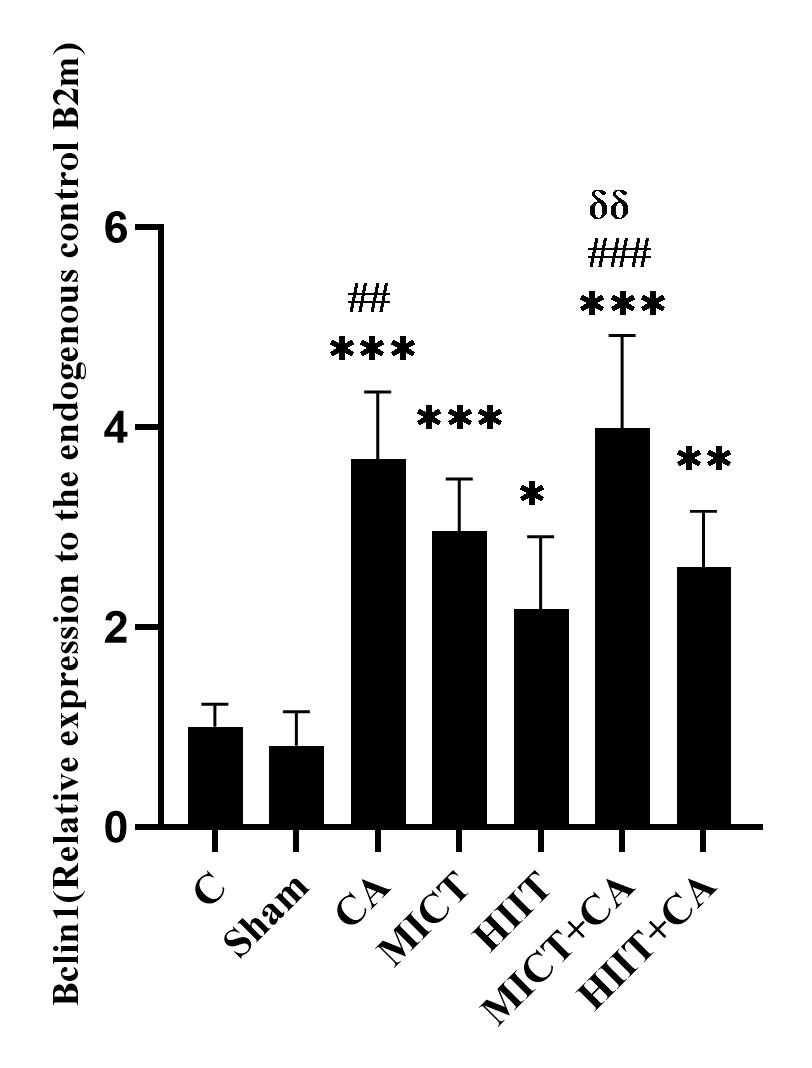
Figure 2. mRNA quantification of Bclin1 gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001), ** (p ≥ 0.01) and * (p ≥ 0.05) Significant increase compared to the C group
### (p ≥ 0.001), ## (p ≥ 0.01) Significant increase compared to the HIIT group
δδ (p ≥ 0.01) Significant increase compared to the HIIT + CA group

Figure 3. mRNA quantification of MyoD gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001) Significant increase compared to the C group.
### (p ≥ 0.001), ## (p ≥ 0.01) Significant increase compared to the CA group
δδδ (p ≥ 0.001) significant increase compared to the MICT + CA group
δ (p ≥ 0.05) significant increase compared to the MICT group
Discussion
Full-Text: (391 Views)
The Effect of Eight Weeks of Continuous and Interval Training with Citrus Aurantium Consumption on Autophagy Markers and MyoD Activation in the Muscle Tissue of Elderly Rats
Mina Jafari 1, Sedigheh Hosseinpour Delavar *1, Hassan Safikhani 2, Masoomeh Azizi 3
Article history
Received 3 Apr 2021
Accepted 14 Sep 2021
A B S T R A C T
Mina Jafari 1, Sedigheh Hosseinpour Delavar *1, Hassan Safikhani 2, Masoomeh Azizi 3
- Department of Exercise Physiology, Kermanshah Branch, Islamic Azad University, Kermanshah, Iran
- Department of Corrective Exercise, School of Physical Education, Kermanshah Branch, Islamic Azad University, Kermanshah, Iran
- Department of Physical Education and Sport Sciences, Islamic Azad University, Abadan Branch, Abadan, Iran
Article history
Received 3 Apr 2021
Accepted 14 Sep 2021
A B S T R A C T
Introduction: Although exercise training and herbs consumption have protective effects on many diseases, the mechanism of action of exercise training with different intensities and citrus aurantium (CA) extract consumption on the autophagy-dependent MyoD activation pathway is not yet known. The aim of this study was to investigate the effect of eight weeks of moderate intensity continuous training (MICT) and high intensity interval training (HIIT) with CA consumption on the expression of LC3-II, Beclin-1 and MyoD as autophagy related markers in the muscle tissue of elderly rats.
Methods: In this experimental study, 42 elderly female rats were randomly assigned to (1) control (C) (2) MICT, (3) HIIT, (4) MICT + CA, (5) HIIT + CA, (6) CA and (7) sham (normal saline) groups. HIIT was performed at 85-110% VO2max intensity and 15-25 m / min speed and MICT at 65% VO2max intensity and 20-25 m / min speed; 300 mg / kg / day CA was received peritoneally. One-way analysis of variance with Tukey's post hoc test was used to analyze the findings. Findings were analyzed using Graph Pad Prism 8.3.0 software (p ≤ 0.05).
Results: MICT and HIIT increased LC3II, Bclin1 and MyoD gene expression (p ≤ 0.05); The effect of HIIT on MyoD increase was greater than MICT (p ≤ 0.05). CA increased the expression of LC3II and Bclin1 (p ≤ 0.05). MICT + CA and HIIT + CA increased the expression of LC3II, Bclin1 and MyoD in the muscle tissue of elderly rats (p ≤ 0.05).
Conclusion: It seems that exercise training and CA consumption with different mechanism of action activate autophagy in the soleus muscle tissue, however the simultaneous use of HIIT, MICT and CA also has favorable effects on the autophagy-dependent MyoD activation pathway.
Keywords: Training, Citrus Aurantium, LC3II, Bclin1, MyoD, Skeletal Muscle
Methods: In this experimental study, 42 elderly female rats were randomly assigned to (1) control (C) (2) MICT, (3) HIIT, (4) MICT + CA, (5) HIIT + CA, (6) CA and (7) sham (normal saline) groups. HIIT was performed at 85-110% VO2max intensity and 15-25 m / min speed and MICT at 65% VO2max intensity and 20-25 m / min speed; 300 mg / kg / day CA was received peritoneally. One-way analysis of variance with Tukey's post hoc test was used to analyze the findings. Findings were analyzed using Graph Pad Prism 8.3.0 software (p ≤ 0.05).
Results: MICT and HIIT increased LC3II, Bclin1 and MyoD gene expression (p ≤ 0.05); The effect of HIIT on MyoD increase was greater than MICT (p ≤ 0.05). CA increased the expression of LC3II and Bclin1 (p ≤ 0.05). MICT + CA and HIIT + CA increased the expression of LC3II, Bclin1 and MyoD in the muscle tissue of elderly rats (p ≤ 0.05).
Conclusion: It seems that exercise training and CA consumption with different mechanism of action activate autophagy in the soleus muscle tissue, however the simultaneous use of HIIT, MICT and CA also has favorable effects on the autophagy-dependent MyoD activation pathway.
Keywords: Training, Citrus Aurantium, LC3II, Bclin1, MyoD, Skeletal Muscle
Copyright © 2021 Elderly Health Journal. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/) which permits copy and redistribute the material just in noncommercial usages, provided the original work is properly cite.
Introduction
The maintenance of muscle mass in the elderly is determined by the dynamics of the balance between anabolic and catabolic processes that change under the influence of pathological conditions (1). In addition to sarcopenia, the loss of muscle mass and age-related decreased muscle function under the physiological process is also associated with chronic disease. This process begins in middle age with an annual loss of 1% of muscle tissue and in more acute conditions reaches over 50% per year between the ages of 80 and 90 (2). From a physiological point of view, sarcopenia is a complex multifactorial mechanism that varies at the levels of mitochondria, motor neurons, myofibrils, and even muscle fibers and has different mechanisms, but one of the important factors in muscle atrophy is the imbalance between protein synthesis and elimination of muscle-dependent proteins and defects in the autophagic mechanism play an important role in its progression (3,4), so that new evidence suggests that defects and reductions in autophagy under catabolic conditions lead to loss of integrity and maintenance of muscle mass (3). In other words, autophagy and proteolysis are processes that occur when the amino acid levels of muscle cells decrease from the mammalian target of rapamycin (mTOR) gene pathway, which leads to the removal of dysfunctional proteins in the myotobules, which results in the development of muscle proteolysis by overexpression of microtubule-associated protein 1 light chain 3 (LC3) following diabetes, cachexia, and malnutrition in skeletal muscle (5). Studies also show that binding of many proteins to the two markers LC3II and Beclin-1 as autophagic markers leads muscle cells to atrophy and apoptosis (6, 7). But because studies show that the increase in mRNA of some myoblast proteins such as Myogenin (MyoG), Myoblast determination protein 1 (MyoD), Myosin heavy chain 3 (Myh3) play a role in the differentiation and proliferation of skeletal muscle, and activation of inactive satellite cells, they are inhibited by an excessive increase in atrogen 5 (ATG5), atrogen 7 (ATG7), LC3II, and Beclin-1 (7).
However, studies show that exercise with the mechanism of activation of metabolic pathways leads to the modulation of autophagy and from this pathway to the activation of satellite cells, mitochondrial biogenesis and the development and improvement of skeletal muscle biological activities (4-5, 8). Exercise appears to activate autophagy pathways with AMP phosphorylation (5); in this regard, the researchers showed that eight weeks, five sessions per week of continuous training increased the expression of ATG7, Beclin-1 and muscle-specific RING finger protein-1 in the lateral lethal muscle (EDL) of elderly rats (8); also, in a study, researchers showed that interval training with an intensity of 40 to 80% of maximum oxygen consumption (VO2max) had more favorable effects on increasing Beclin-1, Atg-1, LC3-II and Atg-12 than continuous training with an intensity of 60% VO2max (9). Eight weeks of resistance training increased Beclin-1, Atg-12, Atg-16 and improved apoptotic markers in elderly men (10). One session of acute exercise also increased the expression of metabolic proteins such as nuclear erythroid-2-p45-related factor-2 (Nrf2) but decreased stem cell population (PAX7) levels and MyoD and increased ubiquitin activity and apoptosis in elderly muscle cells (11). But recent studies have shown that activation of autophagy in improvement of skeletal muscle depends on the type of exercise, the intensity of exercise and its duration. Because studies show that endurance training leads to the induction of metabolic adaptations and resistance training leads to adaptations to increase muscle mass; hence, the mechanism of activation of autophagy-regulated satellite cells following exercise is still unknown (5).
Due to the limited information regarding the most effective treatment for muscle atrophy after aging, it seems that the use of proper diet and herbs has favorable effects on the biology of muscle cells. Among these herbs, citrus aurantium consisting of the flavonoids of hesperidin, neohesperidin, naringin, and narirutin, with its antioxidant effects, reduction of inflammatory and apoptotic factors, as well as the beta-adrenergic signal pathway and phosphorylation of protein kinase A, leads to the activation of many biological pathways (12). In addition, studies have shown that citrus aurantium extract improves metabolism of these tissues following metabolic disorders by increasing insulin sensitivity in skeletal and cardiac muscles (13, 14), so that citrus aurantium extract reduced oxidative stress and increased antioxidants in the heart muscle of chromium-induced elderly rats exposed to oxidative stress (13) and cardiac striated muscle cells following hydrogen peroxide-induced cellular damage (13). Consumption of citrus aurantium extract also decreased TNF-α and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) and increased nitric oxide and inositol-3 phosphate in smooth muscle cells (14).
Due to the limited information on the role of the mechanism of activation of satellite cells regulated by autophagy following exercise in the elderly, as well as the protective and antioxidant effects of citrus aurantium extract, no study was found to investigate the interactive effect of high intensity and low intensity interval training with citrus aurantium extract consumption on this mechanism and the differences between them. Therefore, the aim of this study was to evaluate the effect of eight weeks of moderate intensity continuous training (MICT) and high intensity interval training (HIIT) with citrus aurantium (CA) consumption on LC3-II, Beclin-1 and MyoD in the muscle tissue of elderly rats.
Methods
Study design
In this experimental study, 42 elderly rats in the age range of 14 to 18 months (15, 16), weighing approximately 270 to 320 grams were selected from the Laboratory Animal Breeding Center of Marvdasht Branch of Islamic Azad University and were maintained one week in the Sports Physiology Laboratory of this university for adaptation. It should be noted that all over the research protocol, rats were kept in standard conditions in terms of temperature (22 to 24° C), humidity (55 to 65%), 12-hour light-dark cycle in polycarbonate cages with autoclave capability and had free access to water and food. Then, elderly rats were randomly assigned to (1) control (C) (2) MICT, (3) HIIT, (4) MICT + CA, (5) HIIT + CA, (6) CA and (7) sham (solvent of citrus aurantium extract or normal saline) groups. Groups 3 and 5 performed HIIT (at an intensity of 85-110% VO2max and a speed of 25-25 m / min) for eight weeks, five sessions per week and groups 2 and 4 performed MICT (at an intensity of 65% VO2max at a speed of 20-25 m / min) for eight weeks, five sessions per week (17). Also, the groups 4, 5 and 6 received 300 mg/kg/day of citrus aurantium extract peritoneally (14). Forty-eight hours after the last training session, rats were anesthetized using a combination of ketamine (50 mg/kg) and xylazine (20 mg/kg) and their muscle tissue was extracted by laboratory experts and immersed in a nitrogen tank for further evaluation and then transferred to -80˚ C freezer after 10 minutes.
MICT and HIIT protocol
To assess aerobic power, the rats first warmed up for 5 minutes on a treadmill at a speed of 6 m / min and a slope of zero degree, then every 3 minutes, the speed increased by 3 m / min until the animals became exhausted and could no longer continue.
The criterion for reaching VO2max was the inability of the rats to continue the training protocol and three consecutive collisions with the end of the treadmill in a period of 1 minute, so using the running speed, the amount of VO2max was obtained. It is noteworthy that the maximum running speed has a significant and positive relationship with VO2max (18).
Interval and continuous training started with warm-up at the beginning of each session, including running for 3 minutes at an intensity of 10 meters per minute. Then the HIIT groups performed at an intensity of 85-90% VO2max which was equivalent to 7 attempts of 1 minute and speed of 31 meters / minute, and active rest was performed between intervals with 6 attempts and speed of 15 meters / minute in the first week, which gradually increased with an average of 2 meters / minute per week to 10 attempts of 1 minute at a speed of 55 m / min and active rest with 9 attempts of 1 minute (between intervals) at a speed of 25 m / min in the eighth week.
Moderate-intensity continuous training (MICT) started at an intensity of 65% VO2max, which was equivalent to a speed of 20 m / min and duration of 15 minutes in the first week, which gradually reached a speed of 25 m / min and duration of 31 minutes in the eighth week. The training started with warm up for 3 minutes at an intensity of 10 meters per minute and 2 minutes at an intensity of 15 meters and cooling was performed for 1 minute at an intensity of 15 meters per minute and ended in 2 minutes at an intensity of 10 meters per minute (17).
Evaluation of mRNA of LC3-II, Beclin and MyoD in soleus muscle tissue
For molecular studies on the level of gene expression, first RNA was extracted from the soleus muscle tissue according to the protocol of the manufacturing company (Sinagen, Iran), then the quantity and quality of obtained RNA were checked by measuring the ratio of optical density of 260/280 nm using Nanodrop™ spectrophotometer (Nanodrop; Thermo Fisher Scientific, Wilmington, DE, USA) and then was stored at 80˚C until cDNA synthesis.
After extracting RNA with very high purity and concentration from all studied samples, cDNA synthesis steps were performed according to the manufacturer's protocol and then the synthesized cDNA was used for reverse transcription reaction. First, the designed primers related to genes were examined, and then the expression of genes was examined using quantitative q-Real Time PCR. After completing the activity of the device and observing the graphs based on increasing the number of desired fragments and the amount of fluorescence propagation, by calculating ΔΔCt, the amount of change in the expression of the desired gene compared to B2m and the control group was calculated using the following formula. The sequence of primers used in the study is presented in Table 1.
Ethical considrations
All ethical principles of working with laboratory animals were regarded based on the Helsinki Declaration, and the study was carried out under the supervision of the ethical committee of working with laboratory animals of Kermanshah of Medical Sciences with the approved code of ethics IR.KUMS.REC.1399.412.
Data analysis
The Kolmogorov-Smirnov test was used to evaluate the normality of data distribution. One-way analysis of variance with Tukey’s post hoc test was used to evaluate the findings. Data analysis and plotting of research figures were performed using Graph Pad Prism 8.3.0 software. Also, the significance level was considered 0.05 for all tests.
 Ct= Ct interets- Ct B2m
Ct= Ct interets- Ct B2m
However, studies show that exercise with the mechanism of activation of metabolic pathways leads to the modulation of autophagy and from this pathway to the activation of satellite cells, mitochondrial biogenesis and the development and improvement of skeletal muscle biological activities (4-5, 8). Exercise appears to activate autophagy pathways with AMP phosphorylation (5); in this regard, the researchers showed that eight weeks, five sessions per week of continuous training increased the expression of ATG7, Beclin-1 and muscle-specific RING finger protein-1 in the lateral lethal muscle (EDL) of elderly rats (8); also, in a study, researchers showed that interval training with an intensity of 40 to 80% of maximum oxygen consumption (VO2max) had more favorable effects on increasing Beclin-1, Atg-1, LC3-II and Atg-12 than continuous training with an intensity of 60% VO2max (9). Eight weeks of resistance training increased Beclin-1, Atg-12, Atg-16 and improved apoptotic markers in elderly men (10). One session of acute exercise also increased the expression of metabolic proteins such as nuclear erythroid-2-p45-related factor-2 (Nrf2) but decreased stem cell population (PAX7) levels and MyoD and increased ubiquitin activity and apoptosis in elderly muscle cells (11). But recent studies have shown that activation of autophagy in improvement of skeletal muscle depends on the type of exercise, the intensity of exercise and its duration. Because studies show that endurance training leads to the induction of metabolic adaptations and resistance training leads to adaptations to increase muscle mass; hence, the mechanism of activation of autophagy-regulated satellite cells following exercise is still unknown (5).
Due to the limited information regarding the most effective treatment for muscle atrophy after aging, it seems that the use of proper diet and herbs has favorable effects on the biology of muscle cells. Among these herbs, citrus aurantium consisting of the flavonoids of hesperidin, neohesperidin, naringin, and narirutin, with its antioxidant effects, reduction of inflammatory and apoptotic factors, as well as the beta-adrenergic signal pathway and phosphorylation of protein kinase A, leads to the activation of many biological pathways (12). In addition, studies have shown that citrus aurantium extract improves metabolism of these tissues following metabolic disorders by increasing insulin sensitivity in skeletal and cardiac muscles (13, 14), so that citrus aurantium extract reduced oxidative stress and increased antioxidants in the heart muscle of chromium-induced elderly rats exposed to oxidative stress (13) and cardiac striated muscle cells following hydrogen peroxide-induced cellular damage (13). Consumption of citrus aurantium extract also decreased TNF-α and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) and increased nitric oxide and inositol-3 phosphate in smooth muscle cells (14).
Due to the limited information on the role of the mechanism of activation of satellite cells regulated by autophagy following exercise in the elderly, as well as the protective and antioxidant effects of citrus aurantium extract, no study was found to investigate the interactive effect of high intensity and low intensity interval training with citrus aurantium extract consumption on this mechanism and the differences between them. Therefore, the aim of this study was to evaluate the effect of eight weeks of moderate intensity continuous training (MICT) and high intensity interval training (HIIT) with citrus aurantium (CA) consumption on LC3-II, Beclin-1 and MyoD in the muscle tissue of elderly rats.
Methods
Study design
In this experimental study, 42 elderly rats in the age range of 14 to 18 months (15, 16), weighing approximately 270 to 320 grams were selected from the Laboratory Animal Breeding Center of Marvdasht Branch of Islamic Azad University and were maintained one week in the Sports Physiology Laboratory of this university for adaptation. It should be noted that all over the research protocol, rats were kept in standard conditions in terms of temperature (22 to 24° C), humidity (55 to 65%), 12-hour light-dark cycle in polycarbonate cages with autoclave capability and had free access to water and food. Then, elderly rats were randomly assigned to (1) control (C) (2) MICT, (3) HIIT, (4) MICT + CA, (5) HIIT + CA, (6) CA and (7) sham (solvent of citrus aurantium extract or normal saline) groups. Groups 3 and 5 performed HIIT (at an intensity of 85-110% VO2max and a speed of 25-25 m / min) for eight weeks, five sessions per week and groups 2 and 4 performed MICT (at an intensity of 65% VO2max at a speed of 20-25 m / min) for eight weeks, five sessions per week (17). Also, the groups 4, 5 and 6 received 300 mg/kg/day of citrus aurantium extract peritoneally (14). Forty-eight hours after the last training session, rats were anesthetized using a combination of ketamine (50 mg/kg) and xylazine (20 mg/kg) and their muscle tissue was extracted by laboratory experts and immersed in a nitrogen tank for further evaluation and then transferred to -80˚ C freezer after 10 minutes.
MICT and HIIT protocol
To assess aerobic power, the rats first warmed up for 5 minutes on a treadmill at a speed of 6 m / min and a slope of zero degree, then every 3 minutes, the speed increased by 3 m / min until the animals became exhausted and could no longer continue.
The criterion for reaching VO2max was the inability of the rats to continue the training protocol and three consecutive collisions with the end of the treadmill in a period of 1 minute, so using the running speed, the amount of VO2max was obtained. It is noteworthy that the maximum running speed has a significant and positive relationship with VO2max (18).
Interval and continuous training started with warm-up at the beginning of each session, including running for 3 minutes at an intensity of 10 meters per minute. Then the HIIT groups performed at an intensity of 85-90% VO2max which was equivalent to 7 attempts of 1 minute and speed of 31 meters / minute, and active rest was performed between intervals with 6 attempts and speed of 15 meters / minute in the first week, which gradually increased with an average of 2 meters / minute per week to 10 attempts of 1 minute at a speed of 55 m / min and active rest with 9 attempts of 1 minute (between intervals) at a speed of 25 m / min in the eighth week.
Moderate-intensity continuous training (MICT) started at an intensity of 65% VO2max, which was equivalent to a speed of 20 m / min and duration of 15 minutes in the first week, which gradually reached a speed of 25 m / min and duration of 31 minutes in the eighth week. The training started with warm up for 3 minutes at an intensity of 10 meters per minute and 2 minutes at an intensity of 15 meters and cooling was performed for 1 minute at an intensity of 15 meters per minute and ended in 2 minutes at an intensity of 10 meters per minute (17).
Evaluation of mRNA of LC3-II, Beclin and MyoD in soleus muscle tissue
For molecular studies on the level of gene expression, first RNA was extracted from the soleus muscle tissue according to the protocol of the manufacturing company (Sinagen, Iran), then the quantity and quality of obtained RNA were checked by measuring the ratio of optical density of 260/280 nm using Nanodrop™ spectrophotometer (Nanodrop; Thermo Fisher Scientific, Wilmington, DE, USA) and then was stored at 80˚C until cDNA synthesis.
After extracting RNA with very high purity and concentration from all studied samples, cDNA synthesis steps were performed according to the manufacturer's protocol and then the synthesized cDNA was used for reverse transcription reaction. First, the designed primers related to genes were examined, and then the expression of genes was examined using quantitative q-Real Time PCR. After completing the activity of the device and observing the graphs based on increasing the number of desired fragments and the amount of fluorescence propagation, by calculating ΔΔCt, the amount of change in the expression of the desired gene compared to B2m and the control group was calculated using the following formula. The sequence of primers used in the study is presented in Table 1.
Ethical considrations
All ethical principles of working with laboratory animals were regarded based on the Helsinki Declaration, and the study was carried out under the supervision of the ethical committee of working with laboratory animals of Kermanshah of Medical Sciences with the approved code of ethics IR.KUMS.REC.1399.412.
Data analysis
The Kolmogorov-Smirnov test was used to evaluate the normality of data distribution. One-way analysis of variance with Tukey’s post hoc test was used to evaluate the findings. Data analysis and plotting of research figures were performed using Graph Pad Prism 8.3.0 software. Also, the significance level was considered 0.05 for all tests.
Ct=  Ct Treat-
Ct Treat-  Ct Un Treat
Ct Un Treat
Table 1. The sequence of primers used for measuring research variables
Table 1. The sequence of primers used for measuring research variables
| Genes | Primer Sequences | Sizes (bp) |
| B2m | Forward: 5’- CGTGCTTGCCATTCAGAAA -3’ | 244 |
| Reverse: 5’-ATATACATCGGTCTCGGTGG -3’ | ||
| Beclin 1 | Forward: 5’- AATCTAAGGAGTTGCCGTTATAC-3’ | 187 |
| Reverse: 5’- CCAGTGTCTTCAATCTTGCC -3’ | ||
| LC3II | Forward: 5’- GATCCCAGTGATTATAGAGCGATAC-3’ | 125 |
| Reverse: 5’- GCAGGCGCCTTCTAATTATCT-3’ | ||
| MyoD | Forward: 5’- TGCTCTGATGGCATGATGGATT-3’ | 166 |
| Reverse: 5’- TGGAGATGCGCTCCACTATG -3’ |
Results
Initially, the expression levels of LC3II, Bclin1 and MyoD genes are presented in Figures 1 to 3. The results of one-way analysis of variance showed a significant difference in the levels of LC3II (p ≥ 0.001), Bclin1 (p ≥ 0.001) and MyoD (p ≥ 0.001) in the muscle tissue of elderly rats.
The results of Tukey’s post hoc test showed that there was no significant difference in LC3II gene expression levels in the C and sham groups (p = 0.99), however the levels in the CA (P = 0.001), MICT (p ≥ 0.001), HIIT (p = 0.007), MICT+CA (p ≥ 0.001) and HIIT+CA (p ≥ 0.001) groups were significantly higher than the C group. Also, the levels in the HIIT+CA group were significantly higher than the CA (p ≥ 0.001), MICT (p ≥ 0.001), HIIT (p ≥ 0.001) and MICT+CA (p ≥ 0.001) groups. (Figure 1)
There was no significant difference in Bclin1 levels in the C and sham groups (p = 0.998); however, the levels in the CA (p ≥ 0.001), MICT (p ≥ 0.001), HIIT (p = 0.02), MICT+CA (p ≥ 0.001), HIIT+CA (p ≥ 0.001) groups were significantly higher than the C group.
Bclin1 levels in the CA (p = 0.002) and MICT+CA (P ≥ 0.001) groups were significantly higher than the HIIT group. Also, the levels in the MICT + CA group were significantly higher than the HIIT+CA group (p = 0.005). (Figure 2)
There was no significant difference in MyoD gene expression levels in the C group compared to the sham (p = 0.997) and CA (p = 0.77) groups. But levels in the MICT (p ≥
0.001), HIIT (p ≥ 0.001), MICT+CA (p ≥ 0.001) and HIIT+CA (p ≥ 0.001) groups were higher than the C group. Also, levels in the MICT (p ≥ 0.001), HIIT (p ≥ 0.001), MICT+CA (p = 0.008) and HIIT+CA (p = 0.001) groups were higher than the CA group, and levels in the HIIT group were higher than the MICT (p =0.011) and MICT+CA (p ≥ 0.001) groups. (Figure 3)
The results of Tukey’s post hoc test showed that there was no significant difference in LC3II gene expression levels in the C and sham groups (p = 0.99), however the levels in the CA (P = 0.001), MICT (p ≥ 0.001), HIIT (p = 0.007), MICT+CA (p ≥ 0.001) and HIIT+CA (p ≥ 0.001) groups were significantly higher than the C group. Also, the levels in the HIIT+CA group were significantly higher than the CA (p ≥ 0.001), MICT (p ≥ 0.001), HIIT (p ≥ 0.001) and MICT+CA (p ≥ 0.001) groups. (Figure 1)
There was no significant difference in Bclin1 levels in the C and sham groups (p = 0.998); however, the levels in the CA (p ≥ 0.001), MICT (p ≥ 0.001), HIIT (p = 0.02), MICT+CA (p ≥ 0.001), HIIT+CA (p ≥ 0.001) groups were significantly higher than the C group.
Bclin1 levels in the CA (p = 0.002) and MICT+CA (P ≥ 0.001) groups were significantly higher than the HIIT group. Also, the levels in the MICT + CA group were significantly higher than the HIIT+CA group (p = 0.005). (Figure 2)
There was no significant difference in MyoD gene expression levels in the C group compared to the sham (p = 0.997) and CA (p = 0.77) groups. But levels in the MICT (p ≥
0.001), HIIT (p ≥ 0.001), MICT+CA (p ≥ 0.001) and HIIT+CA (p ≥ 0.001) groups were higher than the C group. Also, levels in the MICT (p ≥ 0.001), HIIT (p ≥ 0.001), MICT+CA (p = 0.008) and HIIT+CA (p = 0.001) groups were higher than the CA group, and levels in the HIIT group were higher than the MICT (p =0.011) and MICT+CA (p ≥ 0.001) groups. (Figure 3)

Figure 1. mRNA quantification of LC3II gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001) Significant increase compared to the C group.
### (p ≥ 0.001) Significant increase compared to the CA, MICT and MICT + CA groups
## (p ≥ 0.01) Significant increase compared to the MICT group

Figure 2. mRNA quantification of Bclin1 gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001), ** (p ≥ 0.01) and * (p ≥ 0.05) Significant increase compared to the C group
### (p ≥ 0.001), ## (p ≥ 0.01) Significant increase compared to the HIIT group
δδ (p ≥ 0.01) Significant increase compared to the HIIT + CA group

Figure 3. mRNA quantification of MyoD gene in soleus muscle of elderly rats, relative to the endogenous control B2m
C: Control, Sham: Citrus aurantium solvent, CA: Citrus Aurantium, MICT: Moderate Intensity Continuous Training, HIIT: High Intensity Interval Training, MICT+CA: Moderate Intensity Continuous Training along with Citrus Aurantium, HIIT+CA: High Intensity Interval Training along with Citrus Aurantium
*** (p ≥ 0.001) Significant increase compared to the C group.
### (p ≥ 0.001), ## (p ≥ 0.01) Significant increase compared to the CA group
δδδ (p ≥ 0.001) significant increase compared to the MICT + CA group
δ (p ≥ 0.05) significant increase compared to the MICT group
Discussion
The results of the present study showed that MICT and HIIT increase the expression of LC3II, Bclin1 and MyoD in the muscle tissue of elderly rats. Also, the effect of HIIT on MyoD increase was more favorable than MICT. Skeletal muscle is believed to depend on the exact coordination of skeletal muscle stem cell regeneration (satellite cells) and immune system activity. However, the decrease in age-related muscle mass with a reduction in regenerative capacity is known as sarcopenia, and it is assumed that one of the factors contributing to the decrease in muscle mass in old age is a decrease in autophagic activity. Recent studies, on the other hand, show that despite different mechanisms in skeletal muscle reduction, autophagy is a catabolic mechanism involved in the regulation and regeneration of satellite cells and myofibrils, so that aging of muscle stem cells is associated with suppression of the autophagic system and loss of cytokines and cellular immune system in the direction of muscle repair (4, 5, 19). In a study by Lee et al., It was stated that the translational regulation of autophagic agents includes the translation of JNK, NFKappaB, HIF-1, and forkhead box O (FOXOs) proteins, and are a key factor in entering the onset phase of autophagy by the mTOR complex (mTORC1 and mTORC2), which activate the autophagosome through specific pathways. Also, the Bclin1: Vps34 complex, which negatively regulates the apoptotic pathways and increases the Bcl-2 family of proteins, promotes autophagosomes to the point that autophagy-dependent proteins Atg5: Atg12 convert LC3I to LC3II. Here, the autophagosome is completed and the complex becomes available to lysosomes, and lysosomal enzymes break down proteins and release new amino acids into the cytosol (19). But studies show that exercise activity and the mechanism of mTOR activation by activating ATGs and the p38α/β pathway of mitogen-activated protein kinase (MAPK), JAK2-STAT3 lead to MyoD activation. Increased MyoD activity is associated with activation of satellite cells, leading to proliferation and increased differentiation of these cells into muscle cells (5,19). However, the phosphorylation of autophagy regulation-related metabolic pathways depends on the intensity and type of exercise. In this regard, the researchers showed that eight weeks of resistance training increased protein levels of Bclin1, Atg12, Atg16 and LAMP-2, decreased the expression of caspase-1 to procaspase-1 ratio and decreased Bad/BcL-2 ratio in elderly men and women (10). In this regard, researchers showed that eight to five sessions per week of continuous training increased the expression of ATG7, Beclin-1 and muscle-specific RING finger protein-1 in the extensor digitorum longus (EDL) of elderly rats (8). Also, in another study, researchers showed that high intensity interval training at an intensity of 40 to 80% VO2max had more favorable effects on increasing Beclin-1, Atg-1, LC3-II and Atg-12 than moderate-intensity continuous training at an intensity of 60% VO2max (9); also, 9 weeks of resistance training improved muscle strength, decreased LC3-II/LC3-I and p62 ratios, as well as increased Beclin 1, Atg5 / 12, Atg7 autophagic markers, cathepsin L lysosomal enzymes, decreased cytochrome-c expression and increased Insulin-like growth factor1 (IGF-1) in the muscle tissue of 18 to 20-month-old rats (20). In a study, researchers showed that HIIT and MICT had beneficial effects on increasing muscle function, muscle strength, muscle fiber size, mitochondrial function, and reducing apoptosis in elderly female rats (21).
Consumption of CA increased the expression of LC3II and Bclin1 in the muscle tissue of elderly rats but had no significant effect on the increase in MyoD gene expression. Citrus aurantium is composed of bioflavonoids that play important roles in cell biology. Researchers have shown that the ingredients of this medicinal plant such as synephrine and pectolinarigenin are the most effective antioxidant, anti-inflammatory and anti-cancer flavonoids. However, studies on the mechanism of action of citrus aurantium on autophagy are very limited and not yet fully understood. On the other hand, researchers believe that CA has beneficial biological effects on cells by modulating autophagy and modulating the AMPK-mTOR signaling pathway and it also inhibits apoptosis. In vivo and in vitro studies have also shown that fructose-derived citric acid and L-malic acid reduce caspase-3 levels (22, 23). Also, beta-adrenergic signaling pathway and phosphorylation of protein kinase A following CA consumption lead to activation of many metabolic pathways and cell repair (12). However, the mechanism of the effect of CA on autophagy is still unknown, but studies also show that 100 and 300 mg / kg CA increase insulin sensitivity in skeletal and heart muscles and improve metabolism, reduce oxidative stress and increase antioxidants in the heart muscle of elderly rats exposed to oxidative damage induced by chromium and hydrogen peroxide (13). Consumption of CA at a dose of 125 to 500 mg / kg decreased TNF-α and NF-KB and increased PI3K, nitric oxide synthetase in smooth muscle cells (14). Dose and duration-dependent consumption of citrus aurantium extract can also eliminate cancer cells, increase autophagy in these cells, increase Beclin 1 and LC3B, and inhibit PI3K / Akt/mTOR signaling pathway and phosphorylation of MAPKs in cancer cells in order to remove these cells (24). Despite many investigations, no study was found to investigate the effect of CA on autophagy in muscle tissue, so the present study was limited in comparing the results of the study with similar studies and hence more studies are needed in this regard.
MICT + CA and HIIT + CA increase the expression of LC3II, Bclin1 and MyoD in the muscle tissue of elderly rats. Studies show that type- and intensity-dependent exercise training with the translational regulation mechanism of JNK, NFKappaB, HIF-1 and FOXOs, modulation of the mTOR complex, enhancement of BTlin1, and enhancement of LC3II leads to breakdown of damaged proteins and release of new amino acids to cytosols (19) and by activating ATGs and p38α / β MAPK pathway, JAK2-STAT3 leads to MyoD activation and then increases the volume and proliferation of myofibrils by activating satellite cells (5,19). CA is also mediated by beta-adrenergic mechanisms, PKA, modulation of AMPK-mTOR signaling pathway, inhibition of apoptosis (22, 23), decreased TNF-α, NF-KB, increased PI3K and increased nitric oxide synthetase in skeletal and smooth muscle cells (14). Studies have been performed on the effect of exercise training with CA consumption, for example, eight weeks of endurance training with synephrine (a CA-derived flavonoid) had no significant effect on improving blood pressure, heart rate and body temperature in rats (25); however, consumption of 975 mg CA and eight weeks of exercise training improved the fat profile and reduced body fat mass in overweight elderly men (26). In a study, researchers showed that taking 100 mg CA improved cardiovascular function and modulated epinephrine and norepinephrine levels following the Wingate test (27). Also, its lack of increase in the CA group can be explained by the fact that muscle damage following sports activities with related mechanisms causes muscle cell response to repair, which this mechanism was not observed in CA consumption.
Conclusion
It appears that exercise training and CA consumption with different mechanisms of action activate autophagy in the soleus muscle tissue, but the simultaneous use of HIIT, MICT and CA consumption also have favorable effects on the autophagy-dependent MyoD activation pathway. According to the results of this study, the use of these interventions (training along with CA) together in human samples is recommended with caution and ethical principles.
Study limitations
Due to the limited information regarding the effect of CA consumption on the activation mechanism of autophagy-dependent satellite cells in muscle cells as well as the cellular and molecular mechanisms of various exercise training, one of the limitations of the present study is the lack of measurement of further physiological variables in this pathway such as ATGs, MAPK, Pax7, mTORs as well as lack of morphological changes and pathology of the soleus tissue in elderly rats. Therefore, it is suggested that in future studies, more variables be measured by different methods.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgment
This article was derived from a doctoral dissertation of psychology at Islamic Azad University, Kermanshah Branch. We would like to thank the All the people who helped us in this research.
Authors’ contributions
All authors have an equal share in this study, have read the manuscript, approved the final version and agreed to be accountable for all aspects of the work.
References
1. Segalés J, Perdiguero E, Serrano AL, Sousa-Victor P, Ortet L, Jardí M, et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nature Communications. 2020; 11(1): 1–13.
2. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews. 2018; 47: 123–32.
3. Rong S, Wang L, Peng Z, Liao Y, Li D, Yang X, et al. The mechanisms and treatments for sarcopenia: could exosomes be a perspective research strategy in the future?. Journal of Cachexia Sarcopenia Muscle. 2020; 11(2): 1-18.
4. Liang J, Zeng Z, Zhang Y, Chen N. Regulatory role of exercise-induced autophagy for sarcopenia. Experimental Gerontology. 2020; 130: 110789.
5. Park SS, Seo Y-K, Kwon K-S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Reports. 2019; 52(1):64.
6. Liu C-W, Huang C-C, Hsu C-F, Li T-H, Tsai Y-L, Lin M-W, et al. SIRT1-dependent mechanisms and effects of resveratrol for amelioration of muscle wasting in NASH mice. BMJ Open Gastroenterology. 2020; 7(1): 1-12.
7. Han S, Cui C, He H, Shen X, Chen Y, Wang Y, et al. FHL1 regulates myoblast differentiation and autophagy through its interaction with LC3. Journal of Cellular Physiology. 2020; 235(5): 4667–78.
8. Kim YA, Kim YS, Oh SL, Kim H-J, Song W. Autophagic response to exercise training in skeletal muscle with age. Journal of Physiology and Biochemistry. 2013; 69(4): 697–705.
9. Weng T-P, Huang S-C, Chuang Y-F, Wang J-S. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One. 2013; 8(11): 1-17.
10. Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging. 2017: 9(2): 408–18.
11. Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, et al. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radical Biology and Medicine. 2014; 71: 402–14.
12. Alfarafisa NM, Kitaguchi K, Yabe T. The aging of skeletal muscle and potential therapeutic effects of extracts from edible and inedible plants. Reviews in Agricultural Science. 2020; 8: 70–88.
13. Chaabane M, Elwej A, Ghorbel I, Boudawara T, Zeghal N, Soudani N. Citrus aurantium L. peel extract mitigates hexavalent chromium-induced oxidative stress and cardiotoxicity in adult rats. Pharmaceutical and Biomedical Research. 2017; 3(2): 8-18.
14. He W, Li Y, Liu M, Yu H, Chen Q, Chen Y, et al. Citrus aurantium L. and its flavonoids regulate TNBS-induced inflammatory bowel disease through anti-inflammation and suppressing isolated jejunum contraction. International Journal of Molecular Sciences. 2018; 19(10): 1-14.
15. Hosseini SA, Salehi O, Keikhosravi F, Hassanpour G, Ardakani HD, Farkhaie F, et al. Mental health benefits of exercise and genistein in elderly rats. Experimental Aging Research. 2021; 1–16.
16. Sengupta P. The laboratory rat: relating its age with human’s. International Journal of Preventive Medicine. 2013; 4(6): 624-30.
17. Yazdanparast Chaharmahali B, Azarbayjani MA, Peeri M, Farzanegi Arkhazloo P. The Effect of moderate and high intensity interval trainings on cardiac apoptosis in the old female rats. Report of Health Care Journal. 2018; 4(1): 26–35.
18. Li F-H, Sun L, Zhu M, Li T, Gao H-E, Wu D-S, et al. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Experimental Gerontology. 2018; 113: 150–62.
19. Lee DE, Bareja A, Bartlett DB, White JP. Autophagy as a therapeutic target to enhance aged muscle regeneration. Cells. 2019; 8(2): 183.
20. Luo L, Lu A-M, Wang Y, Hong A, Chen Y, Hu J, et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Experimental Gerontology. 2013; 48(4): 427–36.
21. Li F-H, Sun L, Wu D-S, Gao H-E, Min Z. Proteomics-based identification of different training adaptations of aged skeletal muscle following long-term high-intensity interval and moderate-intensity continuous training in aged rats. Aging. 2019; 11(12): 4159–82.
22. Ramezannezhad P, Heidari-Soureshjani S, Suhan T. Protective effects of some medicinal plants against myocardial hypoxia. International Journal of Biology and Chemistry. 2019; 12(1): 112–27.
23. Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, Ha SE, Raha S, Lee WS, et al. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018; 10(8): 1-15.
24. Raha S, Yumnam S, Hong GE, Lee HJ, Saralamma VVG, Park H-S, et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. International Journal of Oncology. 2015; 47(3): 1061–9.
25. Hansen DK, George NI, White GE, Abdel-Rahman A, Pellicore LS, Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovascular Toxicology. 2013; 13(3): 208–19.
26. Colker CM, Kaiman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Current Therapeutic Research. 1999; 60(3): 145–53.
27. Kliszczewicz B, Bechke E, Williamson C, Bailey P, Hoffstetter W, McLester J, et al. The influence of citrus aurantium and caffeine complex versus placebo on the cardiac autonomic response: a double blind crossover design. Journal of the International Society of Sports Nutrition. 2018; 15(34): 1–8.
Consumption of CA increased the expression of LC3II and Bclin1 in the muscle tissue of elderly rats but had no significant effect on the increase in MyoD gene expression. Citrus aurantium is composed of bioflavonoids that play important roles in cell biology. Researchers have shown that the ingredients of this medicinal plant such as synephrine and pectolinarigenin are the most effective antioxidant, anti-inflammatory and anti-cancer flavonoids. However, studies on the mechanism of action of citrus aurantium on autophagy are very limited and not yet fully understood. On the other hand, researchers believe that CA has beneficial biological effects on cells by modulating autophagy and modulating the AMPK-mTOR signaling pathway and it also inhibits apoptosis. In vivo and in vitro studies have also shown that fructose-derived citric acid and L-malic acid reduce caspase-3 levels (22, 23). Also, beta-adrenergic signaling pathway and phosphorylation of protein kinase A following CA consumption lead to activation of many metabolic pathways and cell repair (12). However, the mechanism of the effect of CA on autophagy is still unknown, but studies also show that 100 and 300 mg / kg CA increase insulin sensitivity in skeletal and heart muscles and improve metabolism, reduce oxidative stress and increase antioxidants in the heart muscle of elderly rats exposed to oxidative damage induced by chromium and hydrogen peroxide (13). Consumption of CA at a dose of 125 to 500 mg / kg decreased TNF-α and NF-KB and increased PI3K, nitric oxide synthetase in smooth muscle cells (14). Dose and duration-dependent consumption of citrus aurantium extract can also eliminate cancer cells, increase autophagy in these cells, increase Beclin 1 and LC3B, and inhibit PI3K / Akt/mTOR signaling pathway and phosphorylation of MAPKs in cancer cells in order to remove these cells (24). Despite many investigations, no study was found to investigate the effect of CA on autophagy in muscle tissue, so the present study was limited in comparing the results of the study with similar studies and hence more studies are needed in this regard.
MICT + CA and HIIT + CA increase the expression of LC3II, Bclin1 and MyoD in the muscle tissue of elderly rats. Studies show that type- and intensity-dependent exercise training with the translational regulation mechanism of JNK, NFKappaB, HIF-1 and FOXOs, modulation of the mTOR complex, enhancement of BTlin1, and enhancement of LC3II leads to breakdown of damaged proteins and release of new amino acids to cytosols (19) and by activating ATGs and p38α / β MAPK pathway, JAK2-STAT3 leads to MyoD activation and then increases the volume and proliferation of myofibrils by activating satellite cells (5,19). CA is also mediated by beta-adrenergic mechanisms, PKA, modulation of AMPK-mTOR signaling pathway, inhibition of apoptosis (22, 23), decreased TNF-α, NF-KB, increased PI3K and increased nitric oxide synthetase in skeletal and smooth muscle cells (14). Studies have been performed on the effect of exercise training with CA consumption, for example, eight weeks of endurance training with synephrine (a CA-derived flavonoid) had no significant effect on improving blood pressure, heart rate and body temperature in rats (25); however, consumption of 975 mg CA and eight weeks of exercise training improved the fat profile and reduced body fat mass in overweight elderly men (26). In a study, researchers showed that taking 100 mg CA improved cardiovascular function and modulated epinephrine and norepinephrine levels following the Wingate test (27). Also, its lack of increase in the CA group can be explained by the fact that muscle damage following sports activities with related mechanisms causes muscle cell response to repair, which this mechanism was not observed in CA consumption.
Conclusion
It appears that exercise training and CA consumption with different mechanisms of action activate autophagy in the soleus muscle tissue, but the simultaneous use of HIIT, MICT and CA consumption also have favorable effects on the autophagy-dependent MyoD activation pathway. According to the results of this study, the use of these interventions (training along with CA) together in human samples is recommended with caution and ethical principles.
Study limitations
Due to the limited information regarding the effect of CA consumption on the activation mechanism of autophagy-dependent satellite cells in muscle cells as well as the cellular and molecular mechanisms of various exercise training, one of the limitations of the present study is the lack of measurement of further physiological variables in this pathway such as ATGs, MAPK, Pax7, mTORs as well as lack of morphological changes and pathology of the soleus tissue in elderly rats. Therefore, it is suggested that in future studies, more variables be measured by different methods.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgment
This article was derived from a doctoral dissertation of psychology at Islamic Azad University, Kermanshah Branch. We would like to thank the All the people who helped us in this research.
Authors’ contributions
All authors have an equal share in this study, have read the manuscript, approved the final version and agreed to be accountable for all aspects of the work.
References
1. Segalés J, Perdiguero E, Serrano AL, Sousa-Victor P, Ortet L, Jardí M, et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nature Communications. 2020; 11(1): 1–13.
2. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews. 2018; 47: 123–32.
3. Rong S, Wang L, Peng Z, Liao Y, Li D, Yang X, et al. The mechanisms and treatments for sarcopenia: could exosomes be a perspective research strategy in the future?. Journal of Cachexia Sarcopenia Muscle. 2020; 11(2): 1-18.
4. Liang J, Zeng Z, Zhang Y, Chen N. Regulatory role of exercise-induced autophagy for sarcopenia. Experimental Gerontology. 2020; 130: 110789.
5. Park SS, Seo Y-K, Kwon K-S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Reports. 2019; 52(1):64.
6. Liu C-W, Huang C-C, Hsu C-F, Li T-H, Tsai Y-L, Lin M-W, et al. SIRT1-dependent mechanisms and effects of resveratrol for amelioration of muscle wasting in NASH mice. BMJ Open Gastroenterology. 2020; 7(1): 1-12.
7. Han S, Cui C, He H, Shen X, Chen Y, Wang Y, et al. FHL1 regulates myoblast differentiation and autophagy through its interaction with LC3. Journal of Cellular Physiology. 2020; 235(5): 4667–78.
8. Kim YA, Kim YS, Oh SL, Kim H-J, Song W. Autophagic response to exercise training in skeletal muscle with age. Journal of Physiology and Biochemistry. 2013; 69(4): 697–705.
9. Weng T-P, Huang S-C, Chuang Y-F, Wang J-S. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One. 2013; 8(11): 1-17.
10. Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging. 2017: 9(2): 408–18.
11. Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, et al. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radical Biology and Medicine. 2014; 71: 402–14.
12. Alfarafisa NM, Kitaguchi K, Yabe T. The aging of skeletal muscle and potential therapeutic effects of extracts from edible and inedible plants. Reviews in Agricultural Science. 2020; 8: 70–88.
13. Chaabane M, Elwej A, Ghorbel I, Boudawara T, Zeghal N, Soudani N. Citrus aurantium L. peel extract mitigates hexavalent chromium-induced oxidative stress and cardiotoxicity in adult rats. Pharmaceutical and Biomedical Research. 2017; 3(2): 8-18.
14. He W, Li Y, Liu M, Yu H, Chen Q, Chen Y, et al. Citrus aurantium L. and its flavonoids regulate TNBS-induced inflammatory bowel disease through anti-inflammation and suppressing isolated jejunum contraction. International Journal of Molecular Sciences. 2018; 19(10): 1-14.
15. Hosseini SA, Salehi O, Keikhosravi F, Hassanpour G, Ardakani HD, Farkhaie F, et al. Mental health benefits of exercise and genistein in elderly rats. Experimental Aging Research. 2021; 1–16.
16. Sengupta P. The laboratory rat: relating its age with human’s. International Journal of Preventive Medicine. 2013; 4(6): 624-30.
17. Yazdanparast Chaharmahali B, Azarbayjani MA, Peeri M, Farzanegi Arkhazloo P. The Effect of moderate and high intensity interval trainings on cardiac apoptosis in the old female rats. Report of Health Care Journal. 2018; 4(1): 26–35.
18. Li F-H, Sun L, Zhu M, Li T, Gao H-E, Wu D-S, et al. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Experimental Gerontology. 2018; 113: 150–62.
19. Lee DE, Bareja A, Bartlett DB, White JP. Autophagy as a therapeutic target to enhance aged muscle regeneration. Cells. 2019; 8(2): 183.
20. Luo L, Lu A-M, Wang Y, Hong A, Chen Y, Hu J, et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Experimental Gerontology. 2013; 48(4): 427–36.
21. Li F-H, Sun L, Wu D-S, Gao H-E, Min Z. Proteomics-based identification of different training adaptations of aged skeletal muscle following long-term high-intensity interval and moderate-intensity continuous training in aged rats. Aging. 2019; 11(12): 4159–82.
22. Ramezannezhad P, Heidari-Soureshjani S, Suhan T. Protective effects of some medicinal plants against myocardial hypoxia. International Journal of Biology and Chemistry. 2019; 12(1): 112–27.
23. Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, Ha SE, Raha S, Lee WS, et al. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018; 10(8): 1-15.
24. Raha S, Yumnam S, Hong GE, Lee HJ, Saralamma VVG, Park H-S, et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. International Journal of Oncology. 2015; 47(3): 1061–9.
25. Hansen DK, George NI, White GE, Abdel-Rahman A, Pellicore LS, Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovascular Toxicology. 2013; 13(3): 208–19.
26. Colker CM, Kaiman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Current Therapeutic Research. 1999; 60(3): 145–53.
27. Kliszczewicz B, Bechke E, Williamson C, Bailey P, Hoffstetter W, McLester J, et al. The influence of citrus aurantium and caffeine complex versus placebo on the cardiac autonomic response: a double blind crossover design. Journal of the International Society of Sports Nutrition. 2018; 15(34): 1–8.
Type of Study: Research |
Subject:
General
Received: 2021/04/3 | Accepted: 2021/09/14 | Published: 2021/12/19
Received: 2021/04/3 | Accepted: 2021/09/14 | Published: 2021/12/19
References
1. Segalés J, Perdiguero E, Serrano AL, Sousa-Victor P, Ortet L, Jardí M, et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nature Communications. 2020; 11(1): 1–13.
2. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews. 2018; 47: 123–32.
3. Rong S, Wang L, Peng Z, Liao Y, Li D, Yang X, et al. The mechanisms and treatments for sarcopenia: could exosomes be a perspective research strategy in the future?. Journal of Cachexia Sarcopenia Muscle. 2020; 11(2): 1-18.
4. Liang J, Zeng Z, Zhang Y, Chen N. Regulatory role of exercise-induced autophagy for sarcopenia. Experimental Gerontology. 2020; 130: 110789.
5. Park SS, Seo Y-K, Kwon K-S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Reports. 2019; 52(1):64.
6. Liu C-W, Huang C-C, Hsu C-F, Li T-H, Tsai Y-L, Lin M-W, et al. SIRT1-dependent mechanisms and effects of resveratrol for amelioration of muscle wasting in NASH mice. BMJ Open Gastroenterology. 2020; 7(1): 1-12.
7. Han S, Cui C, He H, Shen X, Chen Y, Wang Y, et al. FHL1 regulates myoblast differentiation and autophagy through its interaction with LC3. Journal of Cellular Physiology. 2020; 235(5): 4667–78.
8. Kim YA, Kim YS, Oh SL, Kim H-J, Song W. Autophagic response to exercise training in skeletal muscle with age. Journal of Physiology and Biochemistry. 2013; 69(4): 697–705.
9. Weng T-P, Huang S-C, Chuang Y-F, Wang J-S. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One. 2013; 8(11): 1-17.
10. Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, et al. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging. 2017: 9(2): 408–18.
11. Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, et al. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radical Biology and Medicine. 2014; 71: 402–14.
12. Alfarafisa NM, Kitaguchi K, Yabe T. The aging of skeletal muscle and potential therapeutic effects of extracts from edible and inedible plants. Reviews in Agricultural Science. 2020; 8: 70–88.
13. Chaabane M, Elwej A, Ghorbel I, Boudawara T, Zeghal N, Soudani N. Citrus aurantium L. peel extract mitigates hexavalent chromium-induced oxidative stress and cardiotoxicity in adult rats. Pharmaceutical and Biomedical Research. 2017; 3(2): 8-18.
14. He W, Li Y, Liu M, Yu H, Chen Q, Chen Y, et al. Citrus aurantium L. and its flavonoids regulate TNBS-induced inflammatory bowel disease through anti-inflammation and suppressing isolated jejunum contraction. International Journal of Molecular Sciences. 2018; 19(10): 1-14.
15. Hosseini SA, Salehi O, Keikhosravi F, Hassanpour G, Ardakani HD, Farkhaie F, et al. Mental health benefits of exercise and genistein in elderly rats. Experimental Aging Research. 2021; 1–16.
16. Sengupta P. The laboratory rat: relating its age with human’s. International Journal of Preventive Medicine. 2013; 4(6): 624-30.
17. Yazdanparast Chaharmahali B, Azarbayjani MA, Peeri M, Farzanegi Arkhazloo P. The Effect of moderate and high intensity interval trainings on cardiac apoptosis in the old female rats. Report of Health Care Journal. 2018; 4(1): 26–35.
18. Li F-H, Sun L, Zhu M, Li T, Gao H-E, Wu D-S, et al. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Experimental Gerontology. 2018; 113: 150–62.
19. Lee DE, Bareja A, Bartlett DB, White JP. Autophagy as a therapeutic target to enhance aged muscle regeneration. Cells. 2019; 8(2): 183.
20. Luo L, Lu A-M, Wang Y, Hong A, Chen Y, Hu J, et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Experimental Gerontology. 2013; 48(4): 427–36.
21. Li F-H, Sun L, Wu D-S, Gao H-E, Min Z. Proteomics-based identification of different training adaptations of aged skeletal muscle following long-term high-intensity interval and moderate-intensity continuous training in aged rats. Aging. 2019; 11(12): 4159–82.
22. Ramezannezhad P, Heidari-Soureshjani S, Suhan T. Protective effects of some medicinal plants against myocardial hypoxia. International Journal of Biology and Chemistry. 2019; 12(1): 112–27.
23. Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, Ha SE, Raha S, Lee WS, et al. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018; 10(8): 1-15.
24. Raha S, Yumnam S, Hong GE, Lee HJ, Saralamma VVG, Park H-S, et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. International Journal of Oncology. 2015; 47(3): 1061–9.
25. Hansen DK, George NI, White GE, Abdel-Rahman A, Pellicore LS, Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovascular Toxicology. 2013; 13(3): 208–19.
26. Colker CM, Kaiman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Current Therapeutic Research. 1999; 60(3): 145–53.
27. Kliszczewicz B, Bechke E, Williamson C, Bailey P, Hoffstetter W, McLester J, et al. The influence of citrus aurantium and caffeine complex versus placebo on the cardiac autonomic response: a double blind crossover design. Journal of the International Society of Sports Nutrition. 2018; 15(34): 1–8.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








